ZenQMS for Medical Device Companies
“Our paper-based QMS is impossible to manage.”
We hear you. Meeting regulatory requirements with an outdated system can be overwhelming. That’s why we’re here. ZenQMS was built to help medical device companies like yours stay organized, compliant, and audit-ready, so you can check the boxes and move on with your day.






Selux Diagnostics enjoys fast, trouble-free audits after switching to ZenQMS
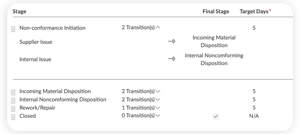
ISSUES MODULE
View your entire risk management process for end-to-end traceability
- Manage CAPAs and non-conformances with just a few clicks
- Design multi-stage workflows, including root cause analysis and effectiveness checks
- Remain 21 CFR 820, ISO 13485, and EU MDR/IVDR compliant
Access all modules from Day 1, including those critical to Medical Device Companies
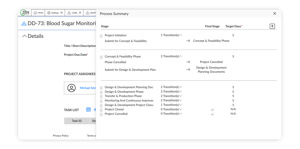
CHANGE CONTROL MODULE
Configure your design control process to match your approach
- Define and build your design control process rather than fitting into a vendor-supplied template
- Import a traceability matrix configured to your preferences
- Access and manage your design history file quickly and easily
- Handle document change requests, ECOs, and electronic batch records more efficiently
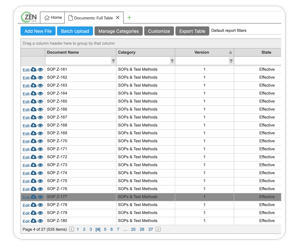
DOCUMENTS MODULE
Create and automate workflows to match your current processes
-
Store and link drawings, work instructions, or documents of any kind and size
-
Version control any type of document (i.e. SOPs, designs, and specs)
-
Stay 21 CFR Part 11/ Annex 11 compliant for all electronic signatures
The ZenQMS Difference
ZenQMS was built on the belief that, regardless of size, every Life Sciences organization should have access to the advantages that come with an electronic quality management system.
Easy-to-use
Rather than force you into pre-designed templates, ZenQMS modules are fully configurable to match your current forms and processes. Familiarity makes things move a lot faster!
Easy-to-Implement
Instead of an arduous 12-month process, we aim to have all clients up and running in 90 days, and often in less time than that. We want you to earn ROI as soon as possible!
Simple, Transparent Pricing
From Day 1, you’ll receive access to every module for everyone on your team. No seat licenses required.
Lifetime Support
You deserve the best help. That's why our customer success team is completely in-house, there's no hourly charge for support, and our response time is measured in minutes.
Peace of Mind
Our system is turn-key and fully managed/supported by ZenQMS-- meaning minimal IT resource needs to maintain it. The cloud-based, closed-loop system ensures data integrity, with guaranteed uptime of >99.99%.
Validation-ready
The ZenQMS platform is fully validated for GxP per GAMP 5 guidelines for a Category 4 system. We are also ISO 9001:2015 and ISO 27001:2022 certified, and HIPAA compliant.
Empathy
We built our platform to identify and address the very same problems and frustrations we encountered as Quality Leaders ourselves. We know what you’re going through.
A Complete System
Right from the start, you have access to all of our connected modules: Documents, Training, Issues, Change Control, and Audits. Implement them at your own pace.
